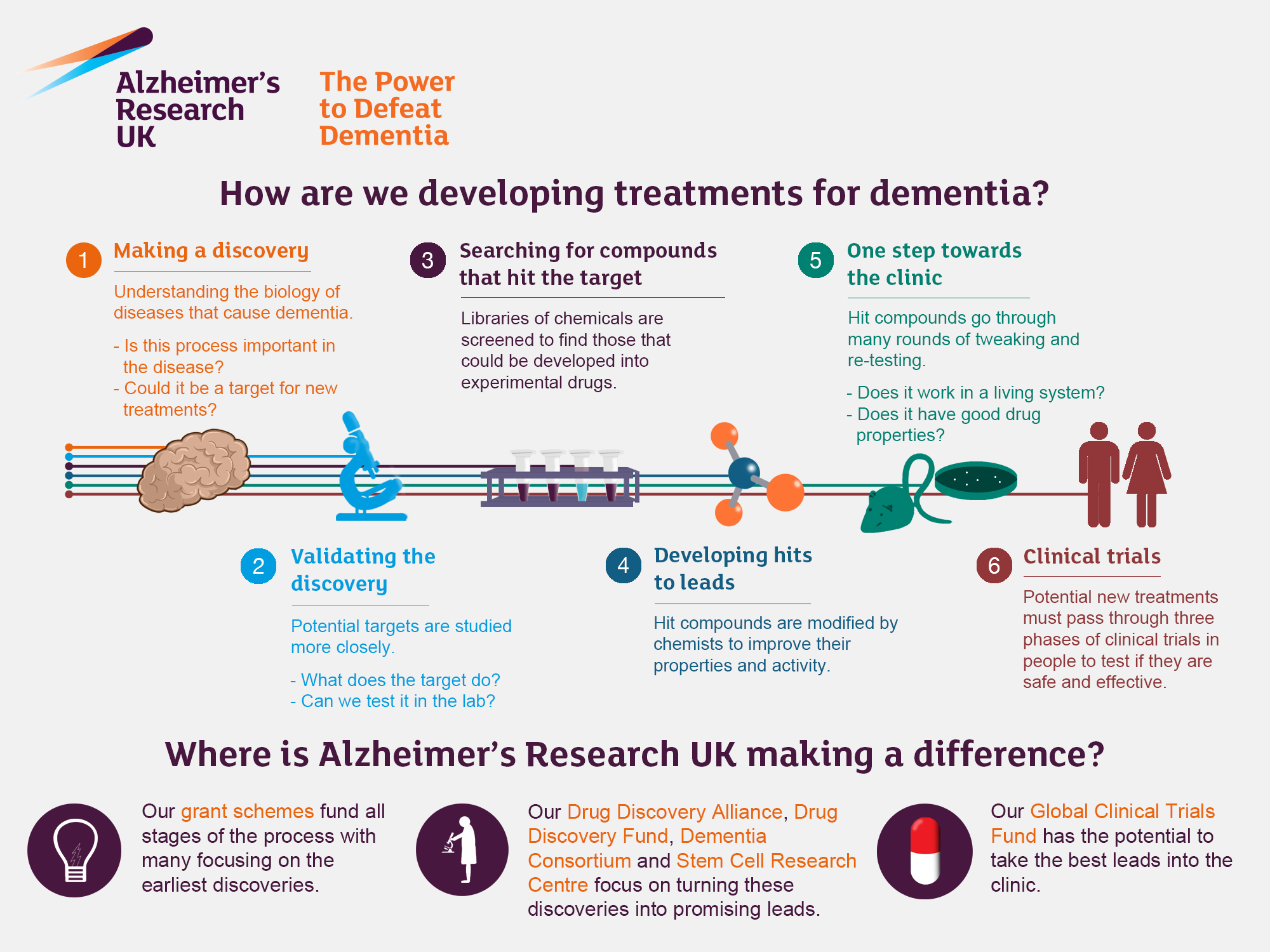
Alzheimer’s Research: Transforming Treatments and Discoveries
Alzheimer’s research is at the forefront of medical science, highlighting groundbreaking discoveries that could transform our understanding of this devastating disease. Leading the charge is neuroscientist Beth Stevens, whose work on microglial cells demonstrates their crucial role in the brain’s immune system and their impact on neurodegenerative diseases like Alzheimer’s. By examining how these cells manage synaptic pruning, Stevens has unveiled potential biomarkers for Alzheimer’s that could enable earlier detection and more effective treatments. With approximately 7 million Americans currently living with Alzheimer’s, her research holds the promise of paving the way toward innovative Alzheimer’s treatment options that could significantly improve the quality of life for patients and their families. As we confront an increasingly aging population, the need for advanced strategies to combat Alzheimer’s has never been more urgent.
Explorations into Alzheimer’s disease encompass a broad spectrum of scientific inquiry aimed at understanding cognitive decline and memory loss in aging populations. The critical study of brain health interfaces with immune responses activated by microglial activity, illustrating their dual role in both maintaining and impairing neural networks. As researchers like Beth Stevens delve into the complexities of neurodegeneration, they unveil important insights into biomarkers that could flag early signs of cognitive impairment. This essential line of investigation not only seeks to identify potential interventions for conditions like Alzheimer’s but also emphasizes the role of federal support in advancing innovative therapies. Altogether, these efforts reinforce the necessity of interdisciplinary research in combating the growing challenge posed by neurodegenerative disorders.
The Role of Microglial Cells in Alzheimer’s Research
Microglial cells are fundamental components of the central nervous system, acting as the brain’s immune defenders. In the context of Alzheimer’s research, these cells play a dual role; they are essential for maintaining neuronal health while also being implicated in neurodegenerative processes when they malfunction. Studies have shown that dysregulated microglial activity can lead to excessive synaptic pruning, a phenomenon noted in Alzheimer’s pathology. This aberrant pruning can disrupt communication between neurons, contributing significantly to cognitive decline.
In recent years, researchers like Beth Stevens have highlighted how understanding microglial function can open new pathways for Alzheimer’s treatment. By identifying the precise mechanisms through which microglial cells operate, scientists can develop targeted therapies aimed at regulating their activity. As we deepen our understanding of these cells, the potential for developing effective biomarkers for Alzheimer’s rises, enabling earlier diagnosis and intervention, which is crucial as the aging population continues to grow.
Innovations in Alzheimer’s Treatment Through Basic Science
The transformative research conducted by Beth Stevens emphasizes the importance of basic science in the quest for Alzheimer’s treatment. By exploring the intricate relationship between microglial cells and synaptic health, Stevens has provided insights that shape our current understanding of neurodegenerative diseases. The foundational knowledge gained from such studies is what allows researchers to develop innovative treatment strategies that could eventually lead to significant improvements in patient care.
Moreover, Stevens’ work exemplifies how federally funded research can lead to groundbreaking discoveries. The support from the National Institutes of Health has not only fueled exploratory research but also positioned scientists like Stevens at the forefront of neuroscience. As new synergies between basic science and clinical applications emerge, we can begin to see the potential for novel therapies that could mitigate the impact of Alzheimer’s disease on millions of individuals.
Advancements in Biomarkers for Alzheimer’s Detection
The pursuit of reliable biomarkers for Alzheimer’s has gained momentum with findings from research focusing on microglial cells. Understanding the processes by which these cells respond to neurodegeneration can lead to the identification of specific biological markers indicative of disease progression. The development of such biomarkers is essential for creating diagnostic tools that allow for early detection of Alzheimer’s, significantly changing the landscape of treatment and management for patients.
Groundbreaking discoveries in microglial activity could potentially inform the design of blood tests or imaging techniques that accurately reflect neural inflammation or synaptic health. Such advancements would not only enhance our diagnostic capabilities but also tailor Alzheimer’s treatment strategies to individual patient profiles, improving outcomes and paving the way for personalized medicine in neurodegenerative disease treatment.
The Future of Neurodegenerative Disease Research
As the global population ages, neurodegenerative diseases such as Alzheimer’s are becoming increasingly prevalent. The urgency for effective treatments and early detection strategies highlights the need for continued research and innovation. Beth Stevens’ work sheds light on the evolving understanding of neurodegenerative diseases, particularly in how microglial dysfunction can lead to the exacerbation of Alzheimer’s symptoms. This paradigm shift in studying brain immune responses will play a critical role in developing future therapies.
Looking ahead, interdisciplinary approaches that integrate neurology, immunology, and genetics will be essential in tackling the complexities of Alzheimer’s. By leveraging advancements in technology and data analysis, researchers can uncover new insights into the interactions between microglial cells and neuronal health. The proactive exploration of these areas holds the promise of not only unveiling new therapeutic targets but also significantly enhancing the quality of life for millions affected by Alzheimer’s disease.
Beth Stevens: A Pioneer in Alzheimer’s Research
Beth Stevens stands out as a pioneering figure in the realm of Alzheimer’s research, known for her groundbreaking insights into the role of microglial cells. Her journey began with a curiosity-driven approach to understanding brain immunity, which has led to discoveries that challenge traditional perspectives on neurodegeneration. Through her work, Stevens has established a critical link between immune response and the onset of diseases like Alzheimer’s, driving forward the field of neuroscience.
Her recognition as a MacArthur genius also underscores the impact of her contributions to Alzheimer’s treatment. Under Stevens’ leadership, the Stevens Lab has cultivated an environment ripe for innovative thinking and exploration, resulting in significant advancements in understanding how microglial dysfunction may precipitate neurodegenerative ailments. As researchers worldwide look to her findings for inspiration, Stevens remains committed to unraveling the complexities of the brain’s immune system, aiming to transform Alzheimer’s disease treatment.
Neuroinflammation: A Key Factor in Alzheimer’s Disease
Neuroinflammation has emerged as a significant factor in the pathogenesis of Alzheimer’s disease, with microglial cells at the forefront of this process. When activated under pathological conditions, microglia can contribute to harmful inflammation, leading to synaptic damage and neuronal loss. Understanding the mechanisms driving neuroinflammation is crucial for identifying potential therapeutic targets and developing strategies to mitigate its effects on cognitive decline.
As research expands in this area, identifying biomarkers associated with neuroinflammation could greatly enhance early diagnosis and intervention in Alzheimer’s. By developing targeted therapies that modulate microglial activity and reduce inflammation, researchers hope to halt or even reverse the progression of Alzheimer’s disease, ultimately improving patient outcomes. This growing field promises to transform how we approach treatment for neurodegenerative disorders.
Understanding Synaptic Health in Alzheimer’s Mechanisms
The health of synapses is critical to cognitive function, and recent studies have highlighted the role of microglial cells in regulating synaptic integrity. Aberrant pruning of synapses by microglia, as observed in Alzheimer’s, directly correlates with cognitive deficits. This new understanding underscores the importance of synaptic health in the context of neurodegenerative diseases and emphasizes the need for research focused on restoring normal pruning processes.
By uncovering the molecular pathways that govern synaptic remodeling, researchers can begin to identify interventions that protect or restore synapses in Alzheimer’s patients. This line of inquiry holds the promise of not just stabilizing cognitive decline but potentially enhancing cognitive function. Such advancements could revolutionize Alzheimer’s treatment paradigms, emphasizing the restoration of synaptic health as a core objective.
Federal Funding: The Backbone of Alzheimer’s Research
Federal funding has been a cornerstone for the advancement of Alzheimer’s research, enabling pivotal studies that might otherwise remain unfunded. The contributions from institutions like the National Institutes of Health have significantly propelled groundbreaking investigations into microglial cells and their roles in neuroinflammation and synaptic pruning. This influx of resources fosters an environment where innovation and discovery can flourish, allowing scientists to pursue complex questions about Alzheimer’s pathology.
As the burden of Alzheimer’s disease increases, the need for sustained investment in research becomes ever more critical. The collaboration of federal agencies with research institutions like Boston Children’s Hospital and the Broad Institute will continue to drive progression in understanding Alzheimer’s. By prioritizing research funding, we can ensure that scientists like Beth Stevens can continue their vital work, leading to breakthroughs that could alleviate the burden of this devastating disease.
The Impact of Aging Population on Alzheimer’s Research
As the U.S. population ages, the incidence of Alzheimer’s disease is projected to soar, raising significant public health concerns. With estimates predicting that cases could reach up to 14 million by 2050, the urgency for effective research and intervention strategies has never been greater. Understanding the dynamics between aging and Alzheimer’s is essential for tailoring research efforts and addressing the needs of this vulnerable population.
Beth Stevens’ research focuses on how age-related changes in microglial function may contribute to the onset of Alzheimer’s disease. By probing these relationships, researchers hope to unveil age-specific mechanisms that promote neurodegeneration. As we strive to combat the challenges posed by an aging populace, the insights gained from studying microglial cells and their interactions with aging will be pivotal in developing effective Alzheimer’s treatments.
Frequently Asked Questions
How do microglial cells contribute to Alzheimer’s research?
Microglial cells play a crucial role in Alzheimer’s research as they act as the brain’s immune system. They are responsible for monitoring and maintaining the brain’s health by clearing out damaged cells and pruning synapses. Recent studies from researchers like Beth Stevens show that abnormal pruning by microglia may contribute to the onset of Alzheimer’s disease and other neurodegenerative disorders.
What are some new biomarkers for Alzheimer’s discovered in recent research?
Recent research into Alzheimer’s has led to the discovery of new biomarkers that can help in the early detection of the disease. These biomarkers may be linked to the activity of microglial cells and other neurodegenerative indicators, providing a way to identify the disease in its earlier stages, ultimately aiding in more effective Alzheimer’s treatment.
Why is Beth Stevens’ work on microglial cells significant for Alzheimer’s treatment?
Beth Stevens’ work on microglial cells is significant because it provides insights into how these immune cells influence neurodegenerative diseases like Alzheimer’s. Her research suggests that correcting the dysfunctional pruning activity of microglia could lead to new therapeutic strategies and potentially improve treatment options for individuals affected by Alzheimer’s.
What role do neurodegenerative diseases play in Alzheimer’s research?
Neurodegenerative diseases, including Alzheimer’s, are a primary focus in current research efforts. Understanding the mechanisms of these diseases, such as the involvement of microglial cells and the identification of biomarkers for Alzheimer’s, is essential for developing effective treatments and preventative strategies.
How does the aging population impact Alzheimer’s research and treatment strategies?
The aging population significantly impacts Alzheimer’s research and treatment strategies due to the projected increase in Alzheimer’s cases. With an expected doubling of cases by 2050, research efforts like those of Beth Stevens are critical in finding new treatment approaches and identifying biomarkers for Alzheimer’s to better manage care for the growing number of affected individuals.
What are the implications of microglial cell research for future Alzheimer’s therapies?
Research on microglial cells has substantial implications for future Alzheimer’s therapies. By understanding how these cells function and contribute to synaptic pruning, researchers can develop novel therapeutic strategies that target these mechanisms, potentially leading to innovative treatments that halt or slow the progress of Alzheimer’s disease.
How can federal funding influence Alzheimer’s research outcomes?
Federal funding plays a vital role in advancing Alzheimer’s research by providing the necessary resources to explore innovative concepts such as those surrounding microglial cells. Successful research programs, like those led by Beth Stevens, often rely heavily on support from organizations such as the National Institutes of Health, enabling significant discoveries that can transform Alzheimer’s treatment.
| Key Points |
|---|
| Neuroscientist Beth Stevens has transformed thinking about microglial cells, which act as the brain’s immune system. |
| Microglia help clear dead or damaged cells and prune synapses that transmit information among neurons, but can lead to issues if not functioning properly. |
| Aberrant pruning by microglia has been linked to Alzheimer’s disease and other neurodegenerative disorders. |
| Stevens’ research is paving the way for new medicines and biomarkers for the early detection of Alzheimer’s and other diseases. |
| The number of Alzheimer’s cases is expected to double by 2050, significantly impacting healthcare costs. |
| Stevens emphasizes the importance of basic and curiosity-driven science, supported by federal funding, in advancing medical research. |
Summary
Alzheimer’s research continues to evolve, driven by groundbreaking findings from scientists like Beth Stevens. Her work on microglial cells challenges existing beliefs and opens new avenues for understanding and addressing neurodegenerative diseases. As the population ages, the implications of this research become even more critical, highlighting the need for innovative solutions in treating conditions like Alzheimer’s.