Gene Therapy for Hemophilia: New Hope for Patients
Gene therapy for hemophilia is revolutionizing the way patients manage this challenging blood disorder. Hemophilia B, caused by a deficiency in clotting factor IX, has long required patients to rely on regular injections for effective treatment. However, with the recent approval of Hemgenix, a groundbreaking gene therapy, individuals living with hemophilia now have the potential for lasting relief. This innovative therapy is designed to provide significant gene therapy benefits, allowing patients like Terence Blue to experience reduced dependence on traditional hemophilia treatment methods and live life with newfound freedom. As research in gene therapy continues to advance, the prospects for those managing hemophilia are brighter than ever, paving the way for a future where the burden of chronic injections may be a thing of the past.
Exploring alternative approaches to hemophilia management, gene therapy emerges as a game-changer in treating this genetic blood disorder. Known for causing unpredictable bleeding episodes, hemophilia has traditionally been managed through regular administration of clotting factors. However, innovative therapies, such as Hemgenix, are now bringing forth a new wave of possibilities for individuals affected by hemophilia B. By harnessing the power of genetic engineering, patients can potentially reduce their reliance on conventional treatment methods, drastically improving their quality of life. This shift marks a significant leap in the quest for effective solutions for those encountering the daily challenges of living with hemophilia.
Understanding Gene Therapy for Hemophilia
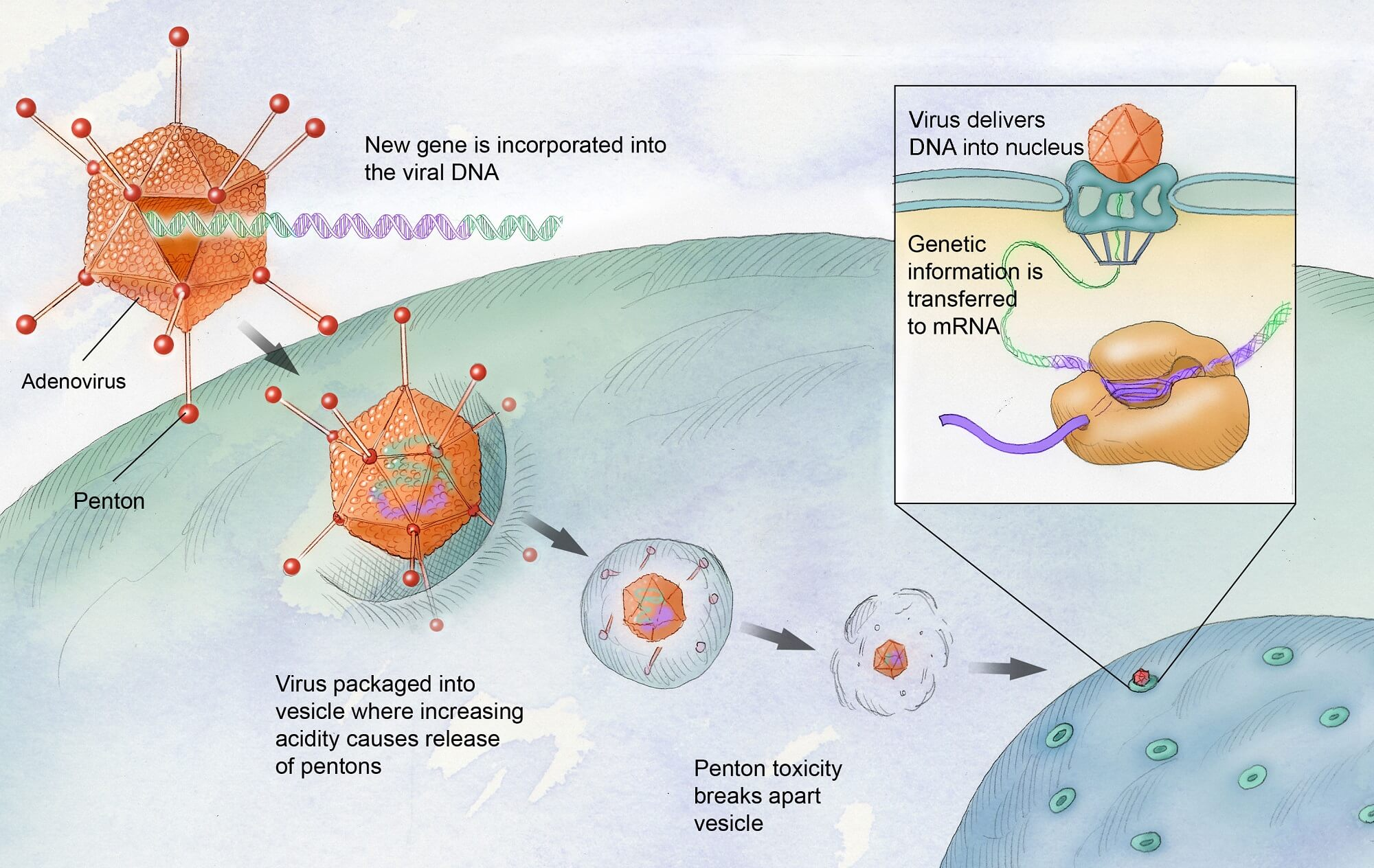
Gene therapy for hemophilia represents a transformative step in medical treatment, aiming to provide a long-lasting solution for individuals suffering from this condition. Hemophilia, particularly hemophilia B, arises from a deficiency in clotting factors, leading to uncontrolled bleeding episodes. Traditional treatment methods, which involve regular injections of clotting factors, can be burdensome and restrictive, hindering the quality of life for many patients. With the emergence of Hemgenix, the first FDA-approved gene therapy for hemophilia B, patients can now hope for a life devoid of the constant anxiety related to bleeding and the painful injections that accompany traditional treatments.
Hemgenix, developed by CSL Behring, uses advanced genetic technology to introduce a correct copy of the mutated gene into the patient’s liver. Once administered, the therapy aims to enable the liver to produce the missing clotting factor IX, significantly reducing or eliminating the need for regular injections. This innovative approach not only offers profound benefits in terms of patient convenience but also promises enhanced well-being. Many patients, including Terence Blue, have reported experiencing an unexpected speed of healing, which can fundamentally change daily living activities and improve life quality for those managing hemophilia.
The Benefits of Hemgenix Gene Therapy
The benefits of gene therapy extend beyond merely addressing the symptoms of hemophilia; they encompass a comprehensive improvement in patients’ overall quality of life. For individuals like Terence Blue, who have lived with hemophilia for decades, Hemgenix offers the tantalizing possibility of reducing treatment burdens. Patients typically spend significant time managing their condition, from frequent hospital visits to self-administration of clotting factors. With gene therapy, the hope is to simplify the treatment landscape, potentially allowing individuals to pursue spontaneous activities without being encumbered by the fear of bleeding episodes.
Moreover, gene therapy has the potential to alleviate the psychological and social challenges associated with living with hemophilia. Many patients encounter stigma or misunderstanding from peers regarding their condition, leading to feelings of isolation. By significantly improving their health outcomes, gene therapy fosters a sense of empowerment and normalcy among patients. They can engage more freely in physical activities and social gatherings, which had previously been sources of anxiety. This holistic enhancement of life, including vast emotional and social dimensions, makes Hemgenix a groundbreaking option in hemophilia treatment.
Living with Hemophilia: Traditional vs. Innovative Treatments
Living with hemophilia can often feel like a balancing act, requiring constant vigilance and management of health. Traditional treatments, while effective, involve a lifestyle where patients must routinely schedule injections and be prepared for potential bleeding episodes. The necessity of carrying emergency supplies and having an ingrained caution during physical activities becomes second nature. Over time, this can lead to an exhausting routine that impacts not just physical health but also emotional wellbeing. Patients like Terence Blue have long navigated this challenging landscape, striving to maintain normalcy amidst the constraints imposed by their condition.
The advent of innovative treatments like Hemgenix is changing the narrative for individuals living with hemophilia. This gene therapy shifts the focus from a reactive approach—responding to bleeding episodes—to a proactive one, empowering patients by potentially eliminating the need for frequent factor treatments. Patients are recognizing the benefits that come with such therapies, as they allow for greater freedom and spontaneity in day-to-day life. With gene therapy, the landscape of living with hemophilia is evolving, opening doors to improved health and wellness, and ultimately enhancing the long-term outlook for those affected.
The Future of Hemophilia Treatment Innovations
The development of Hemgenix marks a significant milestone in the realm of hemophilia treatments, but it is merely the beginning of a broader trend towards innovative therapies. As science continues to advance at a rapid pace, researchers are exploring additional gene therapy options that could cater to more patients and various types of hemophilia. The information gained from the successes and challenges surrounding Hemgenix could pave the way for improved formulations and delivery methods tailored to individual patient needs. This progressive mindset reflects a strong commitment to not only treat but potentially cure genetic disorders influencing the lives of countless individuals.
Moreover, as public awareness and understanding of gene therapies grow, we can expect a paradigm shift in the perception of hemophilia treatment options. Increased education among patients and healthcare providers alike ensures informed decision-making about therapies like Hemgenix. The ultimate goal remains clear: maximizing patient outcomes while minimizing the physical and emotional burdens of hemophilia management. Continued research will likely lead to safer, more effective therapies, thus nurturing optimism among patients who have long awaited revolutionary changes in their treatment, contributing to a future where living with hemophilia can finally resemble a life of freedom.
Market Dynamics Influencing Gene Therapies for Hemophilia
The journey from laboratory research to market availability for gene therapies like Hemgenix reflects not only scientific advancements but also the intricate dance of economics within the healthcare sector. The stark price tag associated with gene therapies—exemplified by Hemgenix’s estimated cost of $3.5 million—poses significant challenges for widespread adoption. Insurance companies play a pivotal role in determining patient access to these innovative treatments. As the market evolves, pricing strategies that balance patient accessibility with the need for research sustainability must be developed to ensure that life-changing therapies continue to reach those in need.
Furthermore, market dynamics are influenced by patient acceptance, which can vary based on individual experiences and perceptions of gene therapies. While the potential benefits are immense, including long-term relief from the burdens of hemophilia management, hesitancy can occur due to concerns about safety or unfamiliarity with new treatment modalities. Therefore, comprehensive education and communication strategies are essential to bridge the knowledge gap and foster acceptance of gene therapies. Stakeholders within the healthcare ecosystem, from pharmaceutical companies to patient advocacy groups, must work together to navigate these challenges and ensure that patients are well-informed and supported throughout their treatment journey.
Exploring Case Studies: Terence Blue’s Journey
Terence Blue’s experience with Hemgenix serves as a compelling case study highlighting the dramatic potential of gene therapy for hemophilia. As a patient who endured years of regular factor IX injections, Blue’s story exemplifies the transformative impact that this therapy can have on an individual’s life. His candid reflections on the burden of daily needles and the emotional toll of constant vigilance underscore the urgent need for innovative treatments. With the administration of Hemgenix, Blue is not just participating in an experimental clinical trial but is also embodying the hope and promise of what modern medicine can achieve.
As Blue navigates his post-treatment life, his journey will be crucial for understanding both the immediate effects of gene therapy and its long-term viability. His rising levels of factor IX and the ability to heal more quickly after injuries illuminate the profound changes that this gene therapy can herald. Patients and healthcare providers alike will watch closely as Blue and others share their experiences, shedding light on practical outcomes as well as the emotional and social implications of living with hemophilia after such a groundbreaking intervention. His journey reflects a larger movement within the hemophilia community, conveying optimism for a future where the burdens of this condition may be significantly lessened.
Overcoming Challenges in Gene Therapy Adoption
Despite the excitement surrounding gene therapy for hemophilia, several intrinsic challenges must be addressed to fully realize its potential. One of the primary obstacles lies in the economic landscape associated with these treatments. Drug manufacturers face immense pressures to recoup significant research and development costs, resulting in high prices for therapies like Hemgenix. Additionally, the market’s ability to sustain itself requires balanced pricing strategies, ensuring that these innovative treatments remain accessible to patients who need them. This urge for sustainability often raises critical questions surrounding healthcare policy and insurance coverage.
Furthermore, there is a pressing need for comprehensive education and outreach initiatives to inform patients about gene therapy options. With innovative options like Hemgenix still relatively new, many patients may be unaware of their potential benefits and how they may change their lives. Overcoming skepticism or hesitancy about new treatments requires effective communication between pharmaceutical companies, physicians, and patient communities to provide transparency regarding the safety and efficacy of these therapies. Only by addressing these challenges can the broader acceptance and implementation of gene therapies for hemophilia be successfully achieved.
The Role of Patient Education in Hemophilia Management
Patient education remains a cornerstone of effective hemophilia management, especially with advancements in treatment options like gene therapy. Educating patients about the nuances of their condition, treatment options, and the implications of new therapies can significantly enhance their treatment experience. It also empowers patients to engage actively with their healthcare providers, fostering conversations that lead to informed decisions regarding their health. In the context of gene therapy, understanding the mechanisms, potential benefits, and risks can help ease patient anxieties, ultimately leading to better health outcomes.
Furthermore, a well-informed patient community is poised to advocate for their needs more effectively. As more individuals like Terence Blue navigate their treatment journeys, sharing experiences and knowledge can cultivate a supportive network. Patient advocacy organizations play a vital role in disseminating accurate information and resources, ensuring that individuals living with hemophilia feel empowered by their treatment choices. By prioritizing education and fostering open dialogue, the healthcare community can better serve the needs of those affected by hemophilia, encouraging a future where innovative treatments become standard practice.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
Gene therapy for hemophilia, specifically for hemophilia B, involves the introduction of a corrected copy of the gene responsible for producing clotting factor IX. This therapy typically uses a modified virus to deliver the gene directly into the patient’s liver cells, enabling them to produce the missing clotting factor and significantly reducing the need for regular hemophilia treatment.
What are the benefits of Hemgenix gene therapy for patients with hemophilia B?
The benefits of Hemgenix gene therapy for hemophilia B include the potential for long-lasting clotting factor production, reducing or eliminating the need for regular injections of clotting factor. Patients can experience fewer bleeding episodes and improved quality of life, such as less anxiety about bleeding and a more active lifestyle.
How does gene therapy for hemophilia differ from traditional hemophilia treatment?
Unlike traditional hemophilia treatment, which typically involves regular infusions of clotting factor, gene therapy aims to correct the underlying genetic defect that causes hemophilia. This means that patients may require only a single treatment to achieve long-term benefits, whereas traditional treatments require ongoing administration.
What are the potential risks of gene therapy for hemophilia?
The potential risks of gene therapy for hemophilia may include immune reactions to the viral vector used to deliver the gene, potential liver toxicity, and uncertainty about the long-term effects of introducing new genetic material into the body. Close monitoring is essential following treatment.
Can gene therapy cure hemophilia completely?
While gene therapy does not guarantee an outright cure for hemophilia, it has shown the potential to significantly improve symptoms and reduce or eliminate the need for prophylactic clotting factor infusions. Many patients treated with Hemgenix have reported long-lasting benefits, suggesting they may live more like individuals without hemophilia.
How is patient eligibility determined for gene therapy for hemophilia?
Patient eligibility for gene therapy for hemophilia is generally determined based on the severity of the condition, previous treatments, overall health, and specific genetic factors. Healthcare providers will assess each patient individually to determine if they are suitable candidates for therapies like Hemgenix.
What can patients expect after receiving gene therapy for hemophilia?
After receiving gene therapy for hemophilia, patients can expect close monitoring for side effects, particularly regarding liver function and immune response. Many report a dramatic reduction in bleeding episodes and an overall improvement in their ability to engage in normal activities without constant worry about managing their condition.
How does gene therapy impact the cost of hemophilia treatment?
While gene therapies like Hemgenix may involve high upfront costs (around $3.5 million), they can potentially reduce long-term expenses associated with chronic hemophilia treatment by decreasing the need for regular clotting factor infusions. This shift could result in significant cost savings over a patient’s lifetime.
What are some experiences of patients living with hemophilia after gene therapy?
Patients living with hemophilia after gene therapy have reported increased freedom from daily needle injections, improved physical health, and a better quality of life. Many express relief and happiness at the prospect of living without the constant management of hemophilia, allowing them to engage more fully in life.
What advancements in gene therapy are anticipated for the future of hemophilia treatment?
Future advancements in gene therapy may include refined techniques for safer and more effective delivery of corrective genes, broader applications for different types of hemophilia, and ongoing clinical trials that explore the durability and efficacy of treatments like Hemgenix, potentially paving the way for more accessible options.
| Key Point | Details |
|---|---|
| Gene Therapy Introduction | Terence Blue received a new gene therapy called Hemgenix, the first in New England, to treat hemophilia B. |
| Background on Hemophilia | Hemophilia is a genetic disorder where blood doesn’t clot properly, often requiring regular injections of clotting factors. |
| Treatment Process | Gene therapy uses a virus to deliver a corrected gene to liver cells, enabling the production of necessary clotting factor IX. |
| Market Implications | High costs of gene therapy (e.g., $3.5 million for Hemgenix) pose challenges for patient access and market sustainability. |
| Patient Experience | After treatment, Blue experienced a significant increase in his clotting factor levels and reported healing faster than ever. |
Summary
Gene therapy for hemophilia represents a groundbreaking advancement in treating this historically challenging condition. Terence Blue’s experience as the first recipient of the Hemgenix therapy illustrates the potential of gene editing to not only alleviate the burdens of frequent injections but also significantly improve quality of life. With advances in medical technology leading to effective treatments, there is hope that gene therapy can provide lasting solutions for individuals affected by hemophilia.